Digital Thread and Med Device
It’s hard to think of an industry more challenging than medical device manufacturing. You face traditional manufacturing challenges (product design and development, go-to-market strategy, supply chain disruption, worker shortages, capacity restraints, etc.) as well as the challenges of modern medicine and the human body, along with stringent regulatory oversight. Success requires creativity, diligence, and speed. Modern med device manufacturers rely heavily on product lifecycle management (PLM) systems to ensure quality of product and regulatory compliance.
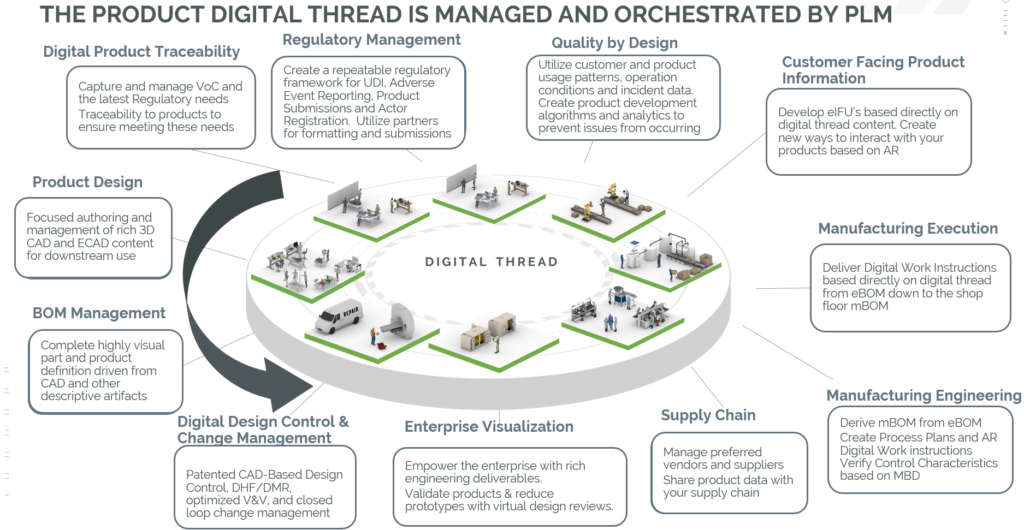
Understanding the concept of a digital thread unlocks understanding of the value of PLM to med device companies. PTC describes a digital thread as “a closed loop between the digital and physical worlds” that transforms how products are designed, manufactured, and serviced. Digital threads should create universal access to data, democratizing engineering data and enabling all teams to participate in the process of product design. Traditionally, the engineering department holds CAD data hostage. With a fully realized digital thread, downstream business units from sales and marketing to quality can access design files. Data is accessible to customers or end users. Imagine the power unlocked by sharing this data so broadly! Security measures are also in place to protect your intellectual property. The digital thread modernizes the sharing of data, and it can be used in unique and broadly effective ways.
For med device manufacturers, a PLM-enabled digital thread combines regulators, manufacturers, and connected product-related data (Internet of Medical Things), resulting in end-to-end visibility. With a fully integrated digital thread, medical device companies can avoid delays due to audits. Legacy systems and file cabinets containing fragmented, analog information can be retired. Instead, all stakeholders connect directly to the current state of the product throughout the entire lifecycle, streamlining change management and regulatory submissions. Medical device PLM truly transforms best practices.
We call the process of building this environment “weaving quality into the digital thread.” It is a true process, and it’s not always easy. Multiple members of your leadership need to buy in: engineering, manufacturing, regulatory and quality, service, and IT. Once complete, a PLM-enabled backbone of quality and compliance can allow your organization to achieve the following:
- Incorporation of risk-based approaches beyond product realization
- Link regulatory requirements with documentation
- Traceability throughout the lifecycle and supply chain
- Harmonization of validation across software applications
- Usability, use of standards, verification and validation planning, design transfer and design records
- Complaint handling and reporting to regulatory authorities in accordance with regulatory requirements
- Post-market surveillance and implementing corrective action without delay
Watch this brief video to learn more about weaving quality into the digital thread using medical device PLM.
Windchill: An Industry Leading Solution
3 HTi is the largest platinum-level PTC reseller on the east coast and Windchill, PTC’s PLM solution, is the industry leader for med device PLM. Windchill provides the foundation and continuity of product and quality information across the digital thread, whether delivered on-premises or SaaS. With a validation-ready cloud, medical device companies and their design/manufacturing partners can take advantage of enterprise and multi-enterprise secure collaboration. Plus, Windchill’s out-of-the-box pre-configured ISO 13485 processes are implemented quickly.
Unsure where to start? 3 HTi also offers a full range of Windchill managed services, from systems audits and diagnostics of your company’s need through white-glove contract systems admin and monitoring services. We understand that a project of this scale can seem daunting, but with over 120 combined years of Windchill expertise and deep experience in the medical device industry, we can guide you through your own digital transformation. Contact us today to get the ball rolling on a diagnostic audit of your company’s PLM and regulatory needs.